Enzymes: Classification, Mechanism of Action and Applications
Enzymes are the biocatalysts. A catalyst is defined as a substance that increases the velocity or rate of a chemical reaction without itself undergoing any change in the overall process.
Enzymes may be defined as biocatalysts synthesized by living cells. They are protein in nature (exception – RNA acting as ribozyme), colloidal and thermolabile in character, and specific in their action.Enzymes are sometimes considered under two broad categories:
- (a) Intracellular enzymes – They are functional within cells where they are synthesized.
- (b) Extracellular enzymes – These enzymes are active outside the cell; all the digestive enzymes belong to this group.
Classification of Enzymes
As per the IUB system of enzyme classification has been in force. Enzymes are divided into six major classes (in that order). Each class on its own represents the general type of reaction brought about by the enzymes of that class.
- 1. Oxidoreductases: Enzymes involved in oxidation-reduction reactions.
- 2. Transferases: Enzymes that catalyse the transfer of functional groups.
- 3. Hydrolases: Enzymes that bring about hydrolysis of various compounds.
- 4. Lyases: Enzymes specialised in the addition or removal of water, ammonia, CO2 etc.
- 5. Isomerases: Enzymes involved in all the isomerization reactions.
- 6. Ligases: Enzymes catalysing the synthetic reactions where two molecules are joined and ATP is used.
Chemical Nature of Enzymes
Enzymes are biological macromolecules made primarily of proteins. They are composed of long chains of amino acids folded into complex 3D shapes after many folding to produce quaternary structure.
This specific shape is essential for their catalytic activity as due to this folding active and binding site are generated which are essential for enzyme activities and for binding of cofactors and coenzymes.The complete enzyme has a protein and non-protein part. The non-protein part is essential for enzymatic activity.
- Apoenzyme: The protein part of an enzyme (inactive alone).
- Cofactor: The non-protein part (can be a metal ion like Mg²⁺, Fe²⁺, Zn²⁺).
- Coenzyme: The non-protein part (an organic molecule e.g., NAD⁺, FAD, coenzyme A)..
- Holoenzyme: The complete, active enzyme (apoenzyme + cofactor).
- Prosthetic Group: The cofactor or coenzyme, which is strongly attached with apoenzyme.
Enzyme Sites
Enzyme has some sites on it, which areActive Site: The active site is the specific region of an enzyme where the substrate binds and the chemical reaction takes place. In other words, it is the site which is responsible for conversion of substrate into product.
Catalytic Site: is a part of active site, as it binds the substrate for conversion, and where the reaction actually takes place.
Binding Site: The binding site is the part of the active site (or sometimes elsewhere or at different place on the enzyme) where the substrate or other molecules (e.g., inhibitors, coenzymes and other biomolecules) attach and affects the enzymatic actions.
Enzyme Inhibition
Enzyme inhibition refers to the decrease in enzyme activity due to the interaction with specific molecules known as inhibitors. This inhibition can be classified as either reversible or irreversible based on the nature of the inhibitor’s interaction with the enzyme.
- Reversible Inhibition: In which the inhibitor binds to the enzyme through non-covalent interactions such as hydrogen bonds, ionic bonds, or hydrophobic forces. This binding is temporary and can be reversed, allowing the enzyme to regain its activity once the inhibitor is removed. Reversible inhibitors are further categorized into competitive, non-competitive, uncompetitive, and allosteric types.
- Irreversible Inhibition: On the other hand, irreversible inhibition involves a permanent inactivation of the enzyme. Irreversible inhibitors typically form covalent bonds with the enzyme, often targeting the active site or a critical functional group necessary for enzymatic activity. This modification permanently alters the enzyme’s structure, rendering it non-functional. Since the enzyme cannot be reactivated, the cell must synthesize new enzyme molecules to restore function.
- Competitive: In competitive inhibition, the inhibitor resembles the substrate and competes for binding at the enzyme’s active site. The inhibitor (I) which closely resembles the real substrate (S) is regarded as a substrate analogue. Because both the substrate and inhibitor cannot bind at the same time, increasing the substrate concentration can overcome the inhibition. This type of inhibition raises the apparent Km (Michaelis constant) but does not affect the maximum reaction velocity (Vmax).
- Non-Competitive: In non-competitive inhibition, the inhibitor binds to a binding site, which is different from the active site—on either the free enzyme or the enzyme-substrate complex. This binding changes the enzyme’s conformation, reducing its catalytic efficiency regardless of substrate concentration. As a result, non-competitive inhibition decreases Vmax, but does not alter Km, because the substrate can still bind with the same affinity but not converted to product.
- Uncompetitive: In this, the inhibitor binds only to the enzyme-substrate complex, not to the free enzyme. This binding typically occurs at a site other than the active site, and this binding of inhibitor and enzyme, stabilizes the enzyme-substrate complex in a way that it prevents the reaction from proceeding i.e. the conversion of substrate into product. Uncompetitive inhibition reduces both Vmax and Km, and unlike competitive inhibition, it cannot be reversed by simply increasing substrate concentration.


Mechanism of Enzyme Activity
Enzymes are biological catalysts that speed up the biochemical reactions by lowering the activation energy required for the reaction to occur.
Activation energy is the minimum amount of energy needed to convert reactants into products. Without enzymes, many biochemical reactions would proceed too slowly due to high activation energy. Enzymes function by binding to the substrate (the reactant molecule) and forming an enzyme-substrate complex. This interaction helps to properly orient the substrate molecules, weaken specific chemical bonds, and stabilize the transition state (Enzyme substrate complex), which is the high-energy intermediate stage of the reaction.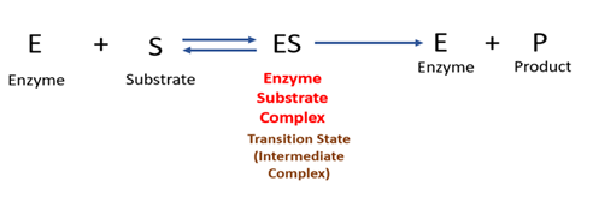 The enzyme may lower the energy of the transition state by creating a charge distribution that is opposite to that of the transition state. This helps lower the activation energy, making it easier for bonds to break and form. By reducing the energy barrier, enzymes allow reactions to proceed more quickly and efficiently under the mild conditions of temperature and pH found in living organisms.
The enzyme may lower the energy of the transition state by creating a charge distribution that is opposite to that of the transition state. This helps lower the activation energy, making it easier for bonds to break and form. By reducing the energy barrier, enzymes allow reactions to proceed more quickly and efficiently under the mild conditions of temperature and pH found in living organisms.

Diagnostic Applications of Enzymes
The diagnostic applications of enzymes refer to the use of specific enzymes as biomarkers to detect, monitor, or confirm diseases. Because enzymes are often released or altered in concentration due to tissue damage or disease, they serve as important indicators of underlying conditions.

Therapeutic Applications of Enzymes
Enzymes are used in treatment of various diseases:
